
Chemistry, 12.09.2019 20:30 owlgirl554
The rate of decomposition of n2o5 in ccl4 at 317 k has been studied by monitoring the concentration of n2o5 in the solution. 2 n2o5(g) → 4 no2(g) + o2(g) initially the concentration of n2o5 is 2.36 m. at 177 minutes, the concentration of n2o5 is reduced to 2.16 m. calculate the average rate of this reaction in m/min.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 04:00, Ezekielcassese
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
The rate of decomposition of n2o5 in ccl4 at 317 k has been studied by monitoring the concentration...
Questions in other subjects:

Mathematics, 18.03.2021 16:50

Mathematics, 18.03.2021 16:50




English, 18.03.2021 16:50


Mathematics, 18.03.2021 16:50


![-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO_{2}]}{\Delta t}=\frac{\Delta [O_{2}]}{\Delta t}](/tpl/images/0229/1014/2f810.png)
![-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}](/tpl/images/0229/1014/bf936.png) represents average rate of disappearance of
represents average rate of disappearance of 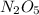 ,
, ![\frac{1}{4}\frac{[NO_{2}]}{\Delta t}](/tpl/images/0229/1014/70f91.png) represents average rate of appearance of
represents average rate of appearance of 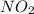 and
and ![\frac{[O_{2}]}{\Delta t}](/tpl/images/0229/1014/eef79.png) represents average rate of appearance of
represents average rate of appearance of 
![-\frac{[N_{2}O_{5}]}{\Delta t}](/tpl/images/0229/1014/beb02.png) =
= 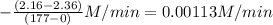
 " alt="-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}" />" /> =
" alt="-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}" />" /> = 


