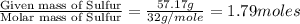In 2006, a russian team discovered an interesting molecule they called "sulflower" because of its shape and because it was based on sulfur. it is composed of 57.17% s and 42.83% c and has a molar mass of 448.70 g/mol. determine the empirical and molecular formulas of "sulflower."

Answers: 3


Other questions on the subject: Chemistry




You know the right answer?
In 2006, a russian team discovered an interesting molecule they called "sulflower" because of its sh...
Questions in other subjects:

Mathematics, 04.07.2020 22:01





Health, 04.07.2020 22:01




 and
and  respectively.
respectively.