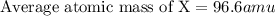An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass of 95.0 amu, 16.90% of isotope x-96 with a mass of 96.0 amu, and 72.42% of isotope x-97 with a mass of 97.0 amu. calculate the atomic mass (in amu) of the element. type in your answer with 3 significant figures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, akatsionis25
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass o...
Questions in other subjects:

History, 01.07.2021 21:20

Mathematics, 01.07.2021 21:20


Mathematics, 01.07.2021 21:20


Mathematics, 01.07.2021 21:20

Biology, 01.07.2021 21:20

Mathematics, 01.07.2021 21:20

English, 01.07.2021 21:20

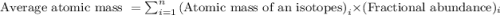 .....(1)
.....(1)![\text{Average atomic mass of X}=[(95\times 0.1068)+(96\times 0.1690)+(97\times 0.7242)]](/tpl/images/0229/1284/93747.png)