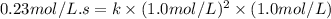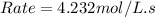Chemistry, 12.09.2019 20:20 juliaduenkelsbu
Enter your answer in the provided box. remember to enter your answer to the correct number of significant figures. for the reaction: a(g) + b(g) → ab(g) the rate is 0.23 mol/l·s, when [a]0 = [b]0 = 1.0 mol/l. if the reaction is first order in b and second order in a, what is the rate when [a]0 = 2.0 mol/l and [b]0 = 4.6 mol/l?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3



Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2
You know the right answer?
Enter your answer in the provided box. remember to enter your answer to the correct number of signif...
Questions in other subjects:




Biology, 16.11.2020 09:10

Biology, 16.11.2020 09:10

Biology, 16.11.2020 09:10

Mathematics, 16.11.2020 09:10

Computers and Technology, 16.11.2020 09:10

English, 16.11.2020 09:10

Mathematics, 16.11.2020 09:10

![Rate=k[A]^2[B]](/tpl/images/0229/0825/4c585.png)