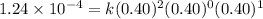Chemistry, 12.09.2019 20:10 ayoismeisjjjjuan
For the reaction a+b+c=> d+e, the initial reaction rate was measured for various initial concentrations of reactants. the following data were collected:
trial a(m) ( c(m) initial rate(m/s)
1 0.40 0.40 0.40 1.2 x 10^-4
2 .40 0.40 .20 .6 x 10^-4
0.80 . 0.40 4.8 x 10^-4
4 0.80 .80 .40 .8 x 10^-4
what is the value of the rate constant k for this reaction?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 14:00, Powerhickory1313
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 15:30, christopherluckey7
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
For the reaction a+b+c=> d+e, the initial reaction rate was measured for various initial concentr...
Questions in other subjects:

History, 01.09.2020 16:01


Geography, 01.09.2020 16:01

English, 01.09.2020 16:01

History, 01.09.2020 16:01

History, 01.09.2020 16:01

Mathematics, 01.09.2020 16:01

Mathematics, 01.09.2020 16:01


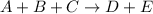
![\text{Rate}=k[A]^a[B]^b[C]^c](/tpl/images/0229/0682/be89a.png)
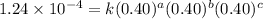 ....(1)
....(1)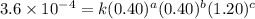 ....(2)
....(2)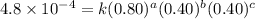 ....(3)
....(3)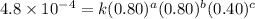 ....(4)
....(4)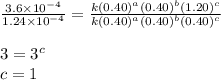
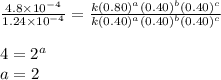
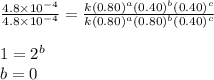
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0229/0682/54afd.png)