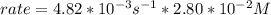Chemistry, 11.09.2019 00:30 nicholasryanencarnac
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at 64 ∘c the rate constant is 4.82 ×10−3s−1. part a part complete write the rate law for the reaction. write the rate law for the reaction. rate=2.41×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5] rate=9.64×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5]2 previous answers correct part b what is the rate of reaction when [n2o5]= 2.80×10−2 m ?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at...
Questions in other subjects:

Mathematics, 29.11.2021 01:20


Mathematics, 29.11.2021 01:30

History, 29.11.2021 01:30


Mathematics, 29.11.2021 01:30

Mathematics, 29.11.2021 01:30


Mathematics, 29.11.2021 01:30

![rate= 4.82*10^{-3}s^{-1} * [N2O5]](/tpl/images/0227/3013/f16e4.png)
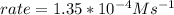
![rate= k * [N2O5]^{x}](/tpl/images/0227/3013/f017a.png)