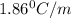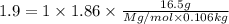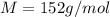Chemistry, 10.09.2019 23:10 blakestuhan
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g of water was found to have a freezing point of -1.9 ∘c. calculate the molar mass of the unknown compound.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1


Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g o...
Questions in other subjects:



Mathematics, 16.12.2020 21:50

Biology, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50



Mathematics, 16.12.2020 21:50

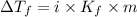
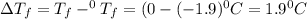 = Depression in freezing point
= Depression in freezing point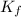 = freezing point constant =
= freezing point constant =