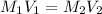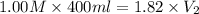Chemistry, 10.09.2019 21:30 hernandez48tur
Achemist must prepare 400 ml of 1.00m aqueous aluminum sulfate working solution. he'll do this by pouring out 1.82 mol/l aqueous aluminum sulfate stock solution into a graduated cylinder and diluting it with distilled water. how many ml of the aluminum sulfate stock solution should the chemist pour out?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

You know the right answer?
Achemist must prepare 400 ml of 1.00m aqueous aluminum sulfate working solution. he'll do this by po...
Questions in other subjects:





History, 02.07.2020 01:01





English, 02.07.2020 01:01

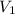 = 400 ml,
= 400 ml, 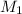 = 1.00 M
= 1.00 M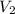 = ?,
= ?, 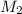 = 1.82 M
= 1.82 M