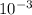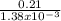The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, with 1 atm o2 pressure is 1.38×10−3 m. part a using henry's law, calculate the molar concentration of o2 in the surface water of a mountain lake saturated with air at 20 ∘c and an atmospheric pressure of 665 torr . express your answer using two significant figures. nothing

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, princessroyal
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 21.06.2019 16:30, TheRealKodakBlack
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1
You know the right answer?
The partial pressure of o2 in air at sea level is 0.21atm. the solubility of o2 in water at 20∘c, wi...
Questions in other subjects:


Mathematics, 17.12.2019 20:31







English, 17.12.2019 20:31

 M
M