
Chemistry, 10.09.2019 18:30 kevinleon695
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas particles have most of their mass concentrated in the nucleus of the atom. b. the moving particles undergo perfectly elastic collisions with the walls of the container. c. the forces of attraction and repulsion between the particles are insignificant. d. the average kinetic energy of the particles is directly proportional to the absolute temperature. e. all of the above are postulates of the kinetic molecular theory.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3


Chemistry, 23.06.2019 23:00, klaytminecraft
What volume of a 2.5 m naoh solution is required to prepare 2.0 liters of a 0.75m naoh? 2.5 liters6.7 liters0.60 liter1.0 liters0.75 liter
Answers: 1
You know the right answer?
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas part...
Questions in other subjects:



Mathematics, 17.02.2021 16:10

Mathematics, 17.02.2021 16:10

Chemistry, 17.02.2021 16:10

Mathematics, 17.02.2021 16:10

Computers and Technology, 17.02.2021 16:10



Mathematics, 17.02.2021 16:20

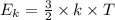 (where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).
(where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).


