
Chemistry, 10.09.2019 18:30 mikayleighb2019
Why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is endothermic by 26.4 kj/mol? why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is endothermic by 26.4 kj/mol? the vapor pressure of the water decreases upon addition of the solute. the osmotic properties of the system lead to this behavior. the overall enthalpy of the system decreases upon addition of the solute. the overall entropy of the system increases upon dissolution of this strong electrolyte. the overall enthalpy of the system increases upon dissolution of this strong electrolyte.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Why does ammonium nitrate (nh4no3) dissolve readily in water even though the dissolution process is...
Questions in other subjects:










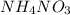 is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (
is ionic in nature. Hence, when it is added to water then it will readily dissociate into ammonium ions (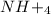 ) and nitrate ions (
) and nitrate ions (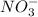 ).
).

