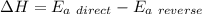The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 218 kj/mol and the change in enthalpy for the reaction is δh = -252 kj/mol . what is the activation energy for the reverse reaction? enter your answer numerically and in terms of kj/mol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1



Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 218 kj/mol and th...
Questions in other subjects:

Mathematics, 12.03.2021 20:20

Mathematics, 12.03.2021 20:20

Mathematics, 12.03.2021 20:20





Mathematics, 12.03.2021 20:20