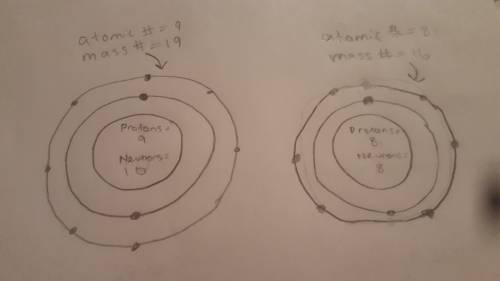
how would the structure of an atom that has an atomic number of 9 and a mass number of 19
diff...

Chemistry, 10.09.2019 04:20 catzdatbloadd
how would the structure of an atom that has an atomic number of 9 and a mass number of 19
differ from the structure of an atom that has an atomic number of 8 and a mass number of 16?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 10.06.2021 18:00



English, 10.06.2021 18:00


History, 10.06.2021 18:00

Arts, 10.06.2021 18:00


Mathematics, 10.06.2021 18:00

Mathematics, 10.06.2021 18:00