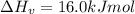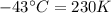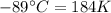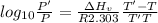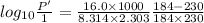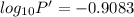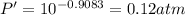Chemistry, 10.09.2019 01:30 natetheman7740
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. calculate the vapor pressure of x at −89.°c. round your answer to 2 significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1


Chemistry, 23.06.2019 03:30, Ramann03
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
The enthalpy of vaporization of substance x is 16.0kj mol and its normal boiling point is −43.°c. ca...
Questions in other subjects:


Mathematics, 11.03.2020 02:27


Mathematics, 11.03.2020 02:27

Mathematics, 11.03.2020 02:27

Biology, 11.03.2020 02:27

Business, 11.03.2020 02:27



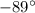 = 0.12 atm
= 0.12 atm