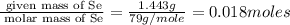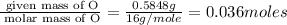Chemistry, 10.09.2019 00:30 chanceypray3514
Consider that a sample of a compound is decomposed and the masses of its constituent elements is as follows: 1.443 g se, 0.5848 g o what would be the empirical formula for this compound?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3


You know the right answer?
Consider that a sample of a compound is decomposed and the masses of its constituent elements is as...
Questions in other subjects:


Biology, 07.04.2021 01:30




Mathematics, 07.04.2021 01:30

History, 07.04.2021 01:30

Mathematics, 07.04.2021 01:30

Mathematics, 07.04.2021 01:30

Mathematics, 07.04.2021 01:30

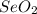 .
.