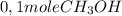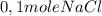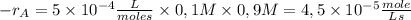Chemistry, 10.09.2019 00:20 disneyshree9427
The reaction described by the equation ch 3 cl + naoh → ch 3 oh + nacl follows the second-order rate law, rate = k [ ch 3 cl ] [ naoh ] . when this reaction is carried out with starting concentrations [ ch 3 cl ] = 0.2 m and [ naoh ] = 1.0 m , the measured rate is 1 × 10 − 4 mol l − 1 s − 1 . what is the rate after one-half of the ch 3 cl has been consumed? (caution: the initial concentrations of the starting materials are not identical in this experiment. hint: determine how much of the naoh has been consumed at this point and what its new concentration is, compared with its initial concentration.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
The reaction described by the equation ch 3 cl + naoh → ch 3 oh + nacl follows the second-order rate...
Questions in other subjects:




Mathematics, 04.10.2019 21:00



Biology, 04.10.2019 21:00

Physics, 04.10.2019 21:00


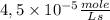
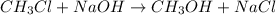
![-r_{A}=k \times [CH_{3}Cl] \times [NaOH]](/tpl/images/0226/2359/ac51f.png)
![1 \times 10^{-4}\frac{mole}{Ls}=k \times [0,2M] \times [1,0M] =5 \times 10^{-4}\frac{L}{mole s}](/tpl/images/0226/2359/a3f49.png)
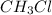 is consumed the mixture is composed by
is consumed the mixture is composed by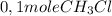 (half is consumed)
(half is consumed)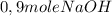 (by stoicheometry)
(by stoicheometry)