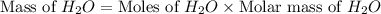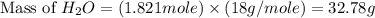Chemistry, 09.09.2019 19:30 Frenchfries13
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 grams of pentane (c5h12) are burned in excess oxygen, how many grams of h2o will be produced?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
Consider the following unbalanced chemical equation. c5h12(l) + o2(g) → co2(g) + h2o(l) if 21.9 gram...
Questions in other subjects:


History, 18.08.2019 22:00

Social Studies, 18.08.2019 22:00

Physics, 18.08.2019 22:00




Mathematics, 18.08.2019 22:00

Mathematics, 18.08.2019 22:00

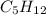 = 21.9 g
= 21.9 g = 18 g/mole
= 18 g/mole

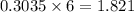 moles of
moles of