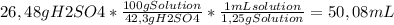Chemistry, 09.09.2019 19:10 runaway173
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by preparing 100 ml of 2.7 m h2so4 using the available solution of 42.3% w/w h2so4. this concentration unit, which may be less familiar to you, is a weight-to-weight percent (100.0 g of the solution contains 42.3 g of h2so4). the density of 42.3% w/w h2so4 is 1.25 g solution/ml solution. using a graduated cylinder, measure out the correct volume of 42.3% w/w h2so4 and slowly add it to a 100-ml volumetric flask that already contains approximately 25 ml of deionized water.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by pre...
Questions in other subjects:

Mathematics, 16.08.2021 21:40

Mathematics, 16.08.2021 21:40

Mathematics, 16.08.2021 21:40

History, 16.08.2021 21:40


History, 16.08.2021 21:40

Chemistry, 16.08.2021 21:40

Mathematics, 16.08.2021 21:40

Social Studies, 16.08.2021 21:40

Mathematics, 16.08.2021 21:40