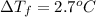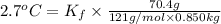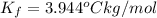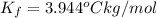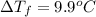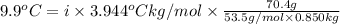Chemistry, 09.09.2019 18:30 chloejaylevesque
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezing point of the solution is 2.7 c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x. calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 09:30, noeliaalvarado
Which element below could be and isotope of this atom
Answers: 1
You know the right answer?
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezi...
Questions in other subjects:

Chemistry, 22.02.2021 23:00

Mathematics, 22.02.2021 23:00



Biology, 22.02.2021 23:00





Mathematics, 22.02.2021 23:00

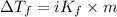
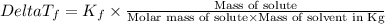 ...(1)
...(1)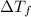 =Elevation in boiling point =
=Elevation in boiling point = 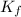 = Freezing point constant
= Freezing point constant