
Chemistry, 13.01.2020 14:31 BookandScienceNerd
Which of the following circumstances will result in a reaction that is spontaneous only at low temperatures?
a.) positive enthalpy change and positive entropy change
b.) negative enthalpy change and negative entropy change
c.) positive enthalpy change and negative entropy change
d.) negative enthalpy change and positive entropy change

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2


Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
Which of the following circumstances will result in a reaction that is spontaneous only at low tempe...
Questions in other subjects:






English, 16.11.2020 17:20

Mathematics, 16.11.2020 17:20


Mathematics, 16.11.2020 17:20


 = Gibbs free energy
= Gibbs free energy 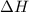 = enthalpy change
= enthalpy change
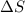 = entropy change
= entropy change 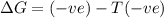
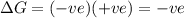 only at low temperature
only at low temperature

