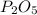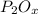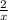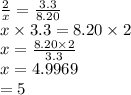An unknown compound has the following chemical formula:
p_2o_x
where x stands for a who...

An unknown compound has the following chemical formula:
p_2o_x
where x stands for a whole number.
measurements also show that a certain sample of the unknown compound contains 8.20 mol of oxygen and 3.3mol of phosphorus.
write the complete chemical formula for the unknown compound.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, Bryanguzman2004
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3


Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 18.03.2021 03:30



Mathematics, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30



Mathematics, 18.03.2021 03:30

Geography, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30