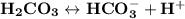Chemistry, 09.09.2019 16:20 shadley6825
One of the buffers that contribute to ph stability in human blood is carbonic acid (h2co3). carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (hco3-) and a hydrogen ion (h ), as noted below. if the ph of blood drops, one would expect one of the buffers that contribute to ph stability in human blood is carbonic acid (h2co3). carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (hco3-) and a hydrogen ion (h ), as noted below. if the ph of blood drops, one would expect the hco3- to act as a base and remove excess h by the formation of h2co3 the concentration of bicarbonate ions (hco3-) to increase the hco3- to act as an acid and remove excess h by the formation of h2co3 a decrease in the concentration of h2co3 and an increase in the concentration of hco3-

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
One of the buffers that contribute to ph stability in human blood is carbonic acid (h2co3). carbonic...
Questions in other subjects: