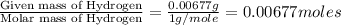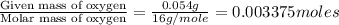Chemistry, 09.09.2019 16:10 batoolishak7475
A0.1014 g sample of a purified compound containing c, h, and, o was burned in a combustion apparatus and produced 0.1486 g co2 and 0.0609 g of h2o. what is the empirical formula of this compound?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1


Chemistry, 23.06.2019 01:00, crysderria
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
A0.1014 g sample of a purified compound containing c, h, and, o was burned in a combustion apparatus...
Questions in other subjects:


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01





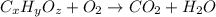


 of carbon will be contained.
of carbon will be contained.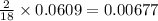 of hydrogen will be contained.
of hydrogen will be contained.