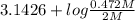Chemistry, 07.09.2019 05:30 brookebeatrice8
Calculate the ph of a solution that is 2.00 m hf, 1.00 m naoh, and 0.472 m naf. (ka=7,2×104)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 21:20, paatnguyyen
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Calculate the ph of a solution that is 2.00 m hf, 1.00 m naoh, and 0.472 m naf. (ka=7,2×104)...
Questions in other subjects:



Mathematics, 04.12.2020 17:40

SAT, 04.12.2020 17:40


English, 04.12.2020 17:40

Mathematics, 04.12.2020 17:40

Physics, 04.12.2020 17:40

Mathematics, 04.12.2020 17:40

 =
= 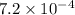
![pK_{a} + log\frac{[salt]}{[acid]}](/tpl/images/0225/1364/8b24d.png)
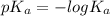
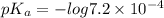
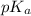 = 3.1426
= 3.1426