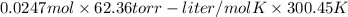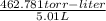Chemistry, 07.09.2019 05:30 dogsarecute278
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas through it at a known temperature and pressure. in an experiment, 5.01 l of n2 gas is passed through 7.9286 g of liquid benzene, c6h6, at 27.3 ∘c and atmospheric pressure. the liquid remaining after the experiment weighs 5.9987 g . assuming that the gas becomes saturated with benzene vapor and that the total gas volume and temperature remain constant, what is the vapor pressure of the benzene in torr?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3
You know the right answer?
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas t...
Questions in other subjects:






Physics, 01.03.2021 22:00


Computers and Technology, 01.03.2021 22:00


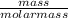
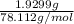
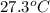 = 27.3 + 273.15 = 300.45 K
= 27.3 + 273.15 = 300.45 K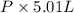 =
=