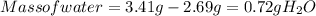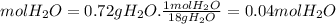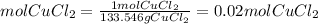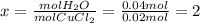Chemistry, 07.09.2019 04:30 batmannn1516
Ahydrate of copper (ii) chloride has the following formula: cucl2 - x h2o. the water in a 3.41-g sample of the hydrate was driven off by heating. the remaining sample had a mass of 2.69 g . find the number of waters of hydration (x) in the hydrate.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Ahydrate of copper (ii) chloride has the following formula: cucl2 - x h2o. the water in a 3.41-g sa...
Questions in other subjects:



History, 29.12.2021 20:00

Computers and Technology, 29.12.2021 20:00



Mathematics, 29.12.2021 20:00

History, 29.12.2021 20:00


History, 29.12.2021 20:00

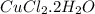
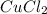 is the mass of remaining sample, because it is a product of loss of drying from initial sample. This means that the mass of water is the mass has been lost.
is the mass of remaining sample, because it is a product of loss of drying from initial sample. This means that the mass of water is the mass has been lost.