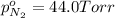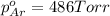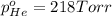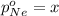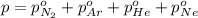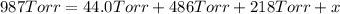Chemistry, 07.09.2019 03:30 rudondo4747
Avessel contained n2, ar, he, and ne. the total pressure in the vessel was 987 torr. the partial pressures of nitrogen, argon, and helium were 44.0, 486, and 218 torr, respectively. the partial pressure of neon in the vessel was torr. a) 42.4 b) 521 c) 19.4 d) 239 e) 760

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, 767sebmont
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2
You know the right answer?
Avessel contained n2, ar, he, and ne. the total pressure in the vessel was 987 torr. the partial pre...
Questions in other subjects:


History, 08.04.2020 20:37




Biology, 08.04.2020 20:37


Mathematics, 08.04.2020 20:37

English, 08.04.2020 20:37

Biology, 08.04.2020 20:37