
Chemistry, 07.09.2019 02:30 sofiisabella10
For a particular reaction, δh∘=67.7 kj/molδh∘=67.7 kj/mol and δ∘=126.9 j/(mol⋅k).δs∘=126.9 j/(mol⋅k). assuming these values change very little with temperature, at what temperature does the reaction change from nonspontaneous to spontaneous in the forward direction?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
For a particular reaction, δh∘=67.7 kj/molδh∘=67.7 kj/mol and δ∘=126.9 j/(mol⋅k).δs∘=126.9 j/(mol⋅k)...
Questions in other subjects:

Health, 28.08.2021 08:30


Biology, 28.08.2021 08:30

Mathematics, 28.08.2021 08:30

English, 28.08.2021 08:30

English, 28.08.2021 08:30


Physics, 28.08.2021 08:30



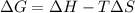
 . If we insert that into our equation we get:
. If we insert that into our equation we get:
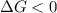 , thus the reaction will be spontaneous.
, thus the reaction will be spontaneous. 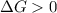 . That is, the reaction will not be spontaneous. Therefore for temperatures higher than 533.49 K we will see a spontaneous reaction, and for temperatures lower than that the reaction will not be spontaneous.
. That is, the reaction will not be spontaneous. Therefore for temperatures higher than 533.49 K we will see a spontaneous reaction, and for temperatures lower than that the reaction will not be spontaneous.


