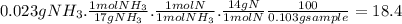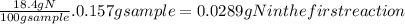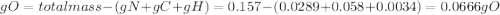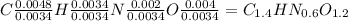Chemistry, 07.09.2019 00:20 blackoak6473
Acompound contains only carbon, hydrogen, nitrogen, and oxygen. combustion of 0.157g of the compound produced 0.213g of co2 and 0.0310g of h2o. in another experiment, it is found that 0.103g of the compound produces 0.0230g of nh3. what is the empirical formula of the compound?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2


Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
Acompound contains only carbon, hydrogen, nitrogen, and oxygen. combustion of 0.157g of the compound...
Questions in other subjects:



English, 31.08.2021 07:10

Mathematics, 31.08.2021 07:10

Spanish, 31.08.2021 07:10

Mathematics, 31.08.2021 07:10

Mathematics, 31.08.2021 07:10

Mathematics, 31.08.2021 07:10

Arts, 31.08.2021 07:10

Physics, 31.08.2021 07:10

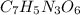
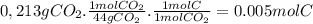
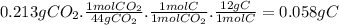
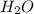 have 0.0034gH or 0.0034mol of H
have 0.0034gH or 0.0034mol of H