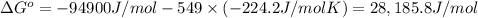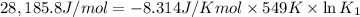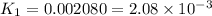Chemistry, 06.09.2019 23:20 zhellyyyyy
Determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh° = -94.9 kj; δs°= -224.2 j/k determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh° = -94.9 kj; δs°= -224.2 j/k 1.07 x 109 481 2.08 x 10-3 9.35 x 10-10 1.94 x 10-12

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3
You know the right answer?
Determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) +...
Questions in other subjects:




Mathematics, 06.01.2022 14:00

Mathematics, 06.01.2022 14:00


Mathematics, 06.01.2022 14:00


Mathematics, 06.01.2022 14:00

History, 06.01.2022 14:00

 .
.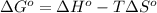
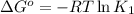
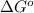 = Gibbs free energy
= Gibbs free energy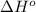 = Enthalpy of reaction
= Enthalpy of reaction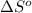 = Entropy of reaction
= Entropy of reaction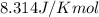
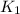 = equilibrium constant at T
= equilibrium constant at T