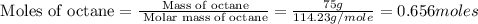Chemistry, 06.09.2019 22:30 amorosoavap5cejz
How much heat is released when 75 g of octane is burned completely if the enthalpy of combustion is -5,500 kj/mol csh18? c8h18 + 25/2 o2 o 8co2 + 9h20 e. 5500 kj a. 7200 kj b. 8360 kj c. 4.1 x 105 kj d. 3600 kj

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, annarain2004
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

You know the right answer?
How much heat is released when 75 g of octane is burned completely if the enthalpy of combustion is...
Questions in other subjects:


Chemistry, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50


Mathematics, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50


Social Studies, 24.02.2021 19:50