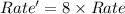Onsider the reaction, no2(g) + co(g) → no(g) + co2(g), for which the rate law has been determined to be: rate = k[no2]2[co]. which of the following statements is/are true? i: the rate of change of no2 is twice the rate of change of co. ii: doubling the concentrations of no2 and co simultaneously will increase the rate of the reaction by a factor of four. a) only ib) only iic) i and iid) none

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1


Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
Onsider the reaction, no2(g) + co(g) → no(g) + co2(g), for which the rate law has been determined to...
Questions in other subjects:

Mathematics, 16.06.2021 18:20

Mathematics, 16.06.2021 18:20


Mathematics, 16.06.2021 18:20

Mathematics, 16.06.2021 18:20



Mathematics, 16.06.2021 18:20


Mathematics, 16.06.2021 18:20

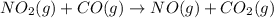
![Rate=k[NO_2]^2[CO]^1](/tpl/images/0224/7810/7e880.png)
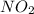 =2
=2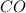 = 1
= 1
![Rate=-\frac{1d[NO_2]}{2dt}=-\frac{1d[CO]}{dt}](/tpl/images/0224/7810/31064.png)
![-\frac{1d[NO_2]}{dt}=-2\times \frac{1d[CO]}{dt}](/tpl/images/0224/7810/6cb34.png)
![Rate'=k[2NO_2]^2[2CO]^1](/tpl/images/0224/7810/f809a.png)
![Rate'=k[2]^2[NO_2]^2[2]^1[CO]^1](/tpl/images/0224/7810/6b7b0.png)
![Rate'=k\times 8[NO_2]^2[2]^1[CO]^1](/tpl/images/0224/7810/be9a6.png)