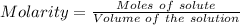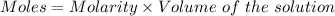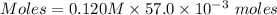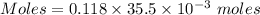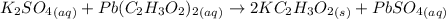Chemistry, 06.09.2019 22:10 loanyst99111
A57.0 ml sample of a 0.120 m potassium sulfate solution is mixed with 35.5 ml of a 0.118 m lead(ii) acetate solution and the following precipitation reaction occurs: k2so4(aq) pb(c2h3o2)2(aq)→2kc2h3o2(aq) pbso4(s) the solid pbso4 is collected, dried, and found to have a mass of 0.992 g . determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
A57.0 ml sample of a 0.120 m potassium sulfate solution is mixed with 35.5 ml of a 0.118 m lead(ii)...
Questions in other subjects:










English, 23.06.2019 02:30