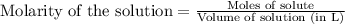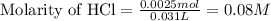Chemistry, 06.09.2019 19:30 hudsonlilliana
What is the molarity of the hydrochloric acid if 31.00 ml of hcl is required to neutralize 0.525 g of sodium carbonate? 2hcl(aq)+na2co3(aq)→2nacl(aq)+h2o(l )+co2(g)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 23.06.2019 03:30, elijahjacksonrp6z2o7
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
What is the molarity of the hydrochloric acid if 31.00 ml of hcl is required to neutralize 0.525 g o...
Questions in other subjects:







Mathematics, 22.07.2020 08:01


Geography, 22.07.2020 08:01

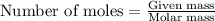
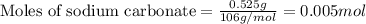
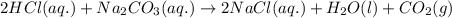
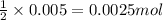 of HCl
of HCl