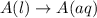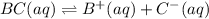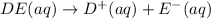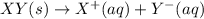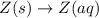Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, marcusajns
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1


Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong...
Questions in other subjects:


Mathematics, 05.11.2020 19:10



Social Studies, 05.11.2020 19:10

Chemistry, 05.11.2020 19:10


Mathematics, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10