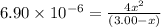Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Consider the reaction. a(aq) = 2 b(aq) kc = 6.90 x 10 6 at 500 k if a 3.00 m sample of a is heated t...
Questions in other subjects:

Mathematics, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20


Mathematics, 10.05.2021 23:20

Mathematics, 10.05.2021 23:20





English, 10.05.2021 23:20

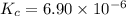
![K_c=\frac{[B]^2}{[A]}](/tpl/images/0224/5358/25ca5.png)