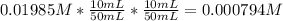Chemistry, 06.09.2019 18:30 angelteddy033
The molar concentration (m) of a solution prepared by dissolving 0.2362g of cr(no3)3 in a 50-ml volumetric flask is 0.01985m, where the molecular weight for cr(no3)3 = 238.01g/mol.
a. suppose you want to prepare another solution containing chromium nitrate that is 25 times less concentrated than the one prepared above. given a choice of 10-ml and 5-ml pipets and 50-ml and 100-ml volumetric flasks, explain how you would proceed in preparing the new diluted solution. in addition, calculate the concentration for the new diluted solution. show all work. your final value should have the correct unit and number of significant figures. hint: you will most likely need two dilution steps in order to obtain the desired concentration. note: you may not reuse the same pipet or combine different pipets within the same dilution step. you may reuse the pipet and/or volumetric flask in the different dilution step.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, hoytkeke6776
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 00:30, bryneosburn
Lem 2 the data below are for the system ethyl propyl ether (1)-chloroform (2) at 0.5 bar. use the data to answer the following questions (all questions refer to p d 0: 5 bar). a) what are the boiling points of the pure components at 0.5 bar? b) a mixture with the overall composition z1 d 0: 1 is brought to 47.6ä±c, 0.5 bar. what is the phase? c) 100 mole of a mixture with z1 d 0: 1 (state a) is mixed with 22 mole of pure ethyl propyl ether vapor (state b). the mixing takes place at 47.6 ä±c, 0.5. bar. what is the phase of the resulting mixture (state c)? if the state is a v/l mixture report the number of moles and mole fractions in each phase. d) plot the txy graph and show states a, b and c. the graph must be done by computer and should be properly annotated. ethyl propyl ether (1) - chloroform (2) at 0.5 bar t ( ä±c) x1 y1 t ( ä±c) x1 y1 42.9 0.000 0.000 49.0 0.470 0.455 43.0 0.020 0.010 49.1 0.520 0.520 43.9 0.065 0.029 48.9 0.567 0.592 45.4 0.156 0.089 48.3 0.652 0.720 46.4 0.215 0.142 47.6 0.745 0.815 47.6 0.296 0.223 46.7 0.822 0.872 48.3 0.362 0.302 45.7 0.907 0.937 48.7 0.410 0.375 44.6 1.000
Answers: 3

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

You know the right answer?
The molar concentration (m) of a solution prepared by dissolving 0.2362g of cr(no3)3 in a 50-ml volu...
Questions in other subjects:


History, 27.09.2020 03:01


History, 27.09.2020 03:01

Mathematics, 27.09.2020 03:01


Mathematics, 27.09.2020 03:01



Mathematics, 27.09.2020 03:01