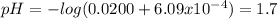A) a solution consists of 0.25 m hydrofluoric acid (hf) and 0.28 m sodium fluoride (naf). the k of hydrofluoric acid acid is 6.8 x 10"", calculate the ph of the solution. b) to one liter of the solution from part (a) is added 0.0200 moles of perchloric acid (hcio4). there is no change in the total volume. calculate the ph after the addition of the perchloric acid.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
A) a solution consists of 0.25 m hydrofluoric acid (hf) and 0.28 m sodium fluoride (naf). the k of h...
Questions in other subjects:

Mathematics, 04.11.2020 02:00

Mathematics, 04.11.2020 02:00

Mathematics, 04.11.2020 02:00



Mathematics, 04.11.2020 02:00

Biology, 04.11.2020 02:00

Mathematics, 04.11.2020 02:00

History, 04.11.2020 02:00

Mathematics, 04.11.2020 02:00

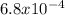 so you can write the equation:
so you can write the equation: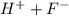
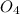 is a strong acid and completely dissociates.
is a strong acid and completely dissociates.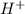 and x moles of
and x moles of 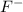 and you can write K like this:
and you can write K like this: ![K=\frac{[H^{+}][F^{-}] }{[HF]}](/tpl/images/0224/4748/809f2.png) . note that
. note that 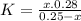 and solving for x, x=6.09x
and solving for x, x=6.09x 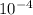 M
M![[H^{+} ]](/tpl/images/0224/4748/cd271.png) and you are abble to find pH with
and you are abble to find pH with ![pH=-log[H^{+}]=-log(6.09x10^{-4} )=3.2](/tpl/images/0224/4748/e6968.png)