Chemistry, 06.09.2019 17:20 aaliyahbaladez56
Aluminum chloride is named as if it is ionic but it is really molecular, with the formula al2cl6. it can be formed by direct reaction of the elements. 2al(s) + 3cl2(g) → aicia) if 0.824 g of aluminum react with excess chlorine, how many grams of aluminum chloride can be obtained? (4.07 g) calculate the grams of chlorine that react with 0.194 g of aluminum. (0.765 g) 1.2.b

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Aluminum chloride is named as if it is ionic but it is really molecular, with the formula al2cl6. it...
Questions in other subjects:



Business, 17.07.2019 06:28

Business, 17.07.2019 06:28

History, 17.07.2019 06:28

Mathematics, 17.07.2019 06:28




Social Studies, 17.07.2019 06:28

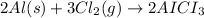
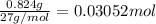
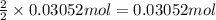 of aluminium chloride.
of aluminium chloride.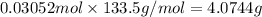
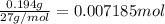
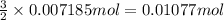 of chlorine gas.
of chlorine gas.