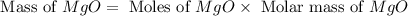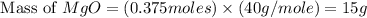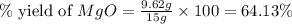Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced equation for the reaction is 2 mg(s) + o2(g) → 2 mgo(s) now consider that you react 10.0 g mg with 6.00 g o2 gas. if you were able to collect 9.62 g of mgo, what would be your percent yield for the reaction?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced e...
Questions in other subjects:




Mathematics, 26.10.2020 09:30

Geography, 26.10.2020 09:30



Mathematics, 26.10.2020 09:30

Engineering, 26.10.2020 09:30

Social Studies, 26.10.2020 09:30

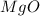 is, 64.13 %
is, 64.13 %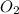 = 6 g
= 6 g

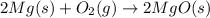
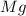
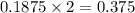 moles of
moles of