Chemistry, 06.09.2019 16:20 glizbethh00
For the following electrochemical reaction: al3+(aq) + 3e -> al(s) eº = -1.66 v e° = 2.87 f2(g) + 2e -> 2f (aq) calculate eº for the cell.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 04:10, nabeelunique
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
For the following electrochemical reaction: al3+(aq) + 3e -> al(s) eº = -1.66 v e° = 2.87 f2(g)...
Questions in other subjects:


History, 30.01.2020 10:59




Mathematics, 30.01.2020 10:59

Chemistry, 30.01.2020 10:59


History, 30.01.2020 10:59

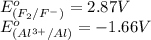
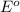 potential will always get reduced and will undergo reduction reaction. Here, fluorine will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, fluorine will undergo reduction reaction will get reduced.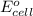 of the reaction, we use the equation:
of the reaction, we use the equation: