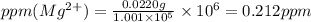Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, irene4523
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 21.06.2019 21:00, maddynichole2017
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2
You know the right answer?
Wastewater from a cement factory contains 0.280 g of ca2+ ion and 0.0220 g of mg2+ ion per 100.0 l o...
Questions in other subjects:

History, 22.09.2019 08:00


History, 22.09.2019 08:00

Mathematics, 22.09.2019 08:00

Mathematics, 22.09.2019 08:00

Biology, 22.09.2019 08:00

Mathematics, 22.09.2019 08:00

Computers and Technology, 22.09.2019 08:00


Biology, 22.09.2019 08:00

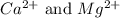 ions are 2.797 ppm and 0.212 ppm respectively.
ions are 2.797 ppm and 0.212 ppm respectively.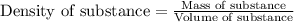
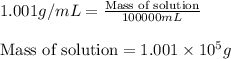
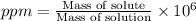
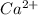 ions = 0.280 g
ions = 0.280 g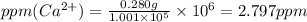
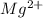 ions = 0.0220 g
ions = 0.0220 g