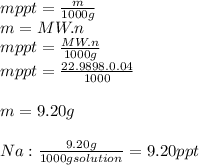
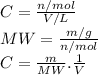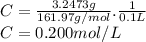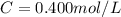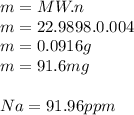3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has a density of 1.00 g/ml, what is the concentration of na (mw = 22.9898 g/mol) in the solution in units of (a) molarity (m)? (b) parts per thousand (ppt)? (c) 10.0 ml of the solution is then diluted to a final volume of 1000.0 ml. what is the concentration of na in the diluted solution in units of parts per million (ppm)?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has...
Questions in other subjects:



English, 28.06.2021 21:00

Mathematics, 28.06.2021 21:00

Mathematics, 28.06.2021 21:00

Mathematics, 28.06.2021 21:00


Mathematics, 28.06.2021 21:00

Chemistry, 28.06.2021 21:00