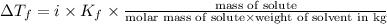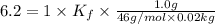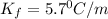Chemistry, 06.09.2019 03:30 ineedtopeebeforethec
Pure nitrobenzene freezes at 5.67 c. when 1.0g of ethanol (c2h6o) is mixed with 20.0g nitrobenzene, the freeze point drops to –0.53 c. what is the freezing-point depression constant (kf) of nitrobenzene?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:20, svaskeacevilles5477
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 06:30, amylumey2005
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
Pure nitrobenzene freezes at 5.67 c. when 1.0g of ethanol (c2h6o) is mixed with 20.0g nitrobenzene,...
Questions in other subjects:





Mathematics, 11.06.2020 18:57

Chemistry, 11.06.2020 18:57


Mathematics, 11.06.2020 18:57

Mathematics, 11.06.2020 18:57

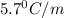
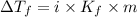
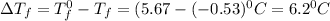 = Depression in freezing point
= Depression in freezing point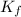 = freezing point constant = ?
= freezing point constant = ?