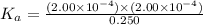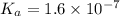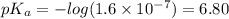Amonoprotic weak acid, ha , dissociates in water according to the reaction ha(aq)↽−−⇀h+(aq)+a−(aq) the equilibrium concentrations of the reactants and products are [ha]=0.250 m , [h+]=2.00×10−4 m , and [a−]=2.00×10−4 m . calculate the value of pka for the acid ha .

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
Amonoprotic weak acid, ha , dissociates in water according to the reaction ha(aq)↽−−⇀h+(aq)+a−(aq) t...
Questions in other subjects:


Mathematics, 10.12.2020 01:00


Chemistry, 10.12.2020 01:00


English, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Health, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

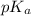 of HA is 6.80
of HA is 6.80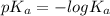
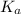 ) of HA is represented as-
) of HA is represented as-![K_{a}=\frac{[H^{+}][A^{-}]}{[HA]}](/tpl/images/0223/8979/c814f.png)