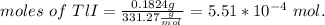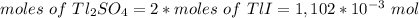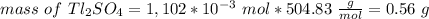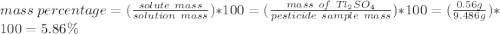Chemistry, 05.09.2019 22:30 Ashley606hernandez
The thallium (present as tl2so4) in a 9.486-g pesticide sample was precipitated as thallium(i) iodide. calculate the mass percent of tl2so4 in the sample if 0.1824 g of tli was recovered.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1



Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
The thallium (present as tl2so4) in a 9.486-g pesticide sample was precipitated as thallium(i) iodid...
Questions in other subjects:






Mathematics, 14.06.2021 15:10

Physics, 14.06.2021 15:10



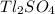 is 5.86%
is 5.86%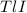 precipitate. We can establish a relation between the mass of
precipitate. We can establish a relation between the mass of