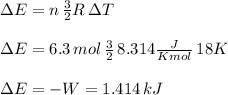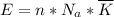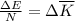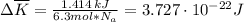Chemistry, 05.09.2019 21:20 jackchelly
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what are (a) the work w done by the gas, (b) the energy transferred as heat q, (c) the change δeint in internal energy of the gas, and (d) the change δk in the average kinetic energy per atom?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 23.06.2019 07:00, tiarafaimealelei
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
You know the right answer?
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what...
Questions in other subjects:



Mathematics, 02.03.2021 17:20



Geography, 02.03.2021 17:20

English, 02.03.2021 17:20

Mathematics, 02.03.2021 17:20

History, 02.03.2021 17:20

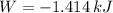 , that means, work is done on the gas, not viceversa.
, that means, work is done on the gas, not viceversa.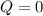 because it is an adiabatic process.
because it is an adiabatic process.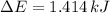
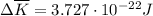 per atom
per atom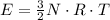 , This result can be experimentally verified or derived from statistical mechanics.
, This result can be experimentally verified or derived from statistical mechanics.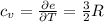
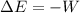
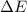 as follows:
as follows: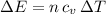 which follows from our first equation.
which follows from our first equation.