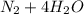Write half-reactions for the oxidation and reduction process for each of the following.
a. fe...

Chemistry, 05.09.2019 20:30 hamadehassan
Write half-reactions for the oxidation and reduction process for each of the following.
a. fe2+ + mno4 - fe3+ + mn2+
b. sn2+ + io3 - sn4+ + i-
c. s2- + no3 - s + no
d. nh3 + no2 n2 + h2o

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 23.06.2019 02:30, babbity2009
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 18:00, den14
Which action would reduce the activation energy of a chemical reaction? a. adding a catalyst to the reaction b. adding more reactants to the reaction vessel c. lowering the potential energy of the products d. lowering the potential energy of the reactants e. raising the temperature of the reaction
Answers: 1
You know the right answer?
Questions in other subjects:


Biology, 23.02.2021 06:00


English, 23.02.2021 06:00


Social Studies, 23.02.2021 06:00

Health, 23.02.2021 06:00

Health, 23.02.2021 06:00


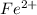 ⇒
⇒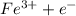
 + 2
+ 2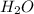 + 3
+ 3 ⇒
⇒ 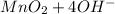
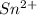 ⇒
⇒ 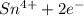
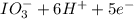 ⇒
⇒ 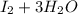
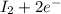 ⇒
⇒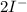
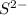 ⇒
⇒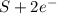
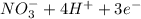 ⇒
⇒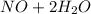
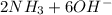 ⇒
⇒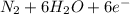
 ⇒
⇒