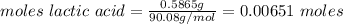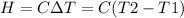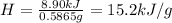Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 23.06.2019 07:00, tiarafaimealelei
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2

Chemistry, 23.06.2019 12:50, vinniemccray70
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1
You know the right answer?
A0.5865-g sample of lactic acid (hc3h5o3) is burned in a calorimeter whose heat capacity is 4.812 kj...
Questions in other subjects:


Social Studies, 26.04.2021 21:00



Physics, 26.04.2021 21:00


Mathematics, 26.04.2021 21:00

English, 26.04.2021 21:00

English, 26.04.2021 21:00