
Chemistry, 05.09.2019 18:30 trillsmith
At equilibrium the reactant and product concentrations are constant because a change in one direction is balanced by a change in the other as the forward and reverse rates become equal: at equilibrium: rate_twd=rate_rev when a chemical system is at equilibrium, a. he concentrations of the reactants and products have reached constant values. b. the concentrations of the reactants are equal to the concentrations of the products. c. the forward and reverse reactions have stopped. d. the reaction quotient, q, has reached a maximum. e. none of the choices.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

You know the right answer?
At equilibrium the reactant and product concentrations are constant because a change in one directio...
Questions in other subjects:

Health, 20.10.2020 22:01


Physics, 20.10.2020 22:01

Spanish, 20.10.2020 22:01



Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

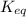
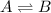
![K_{eq}=\frac{[B]}{[A]}](/tpl/images/0223/5934/89517.png)


